Identifying the Hr-HPV infections that really matter
Proofer 7 HPV-mRNA E6 and E7 Biomarker Test
Our 7 HPV-mRNA E6 and E7 technology (HPV 16, 18, 31, 33, 45, 52, and 58) focuses on the prevention and early detection of cervical cancer.
7 HPV-genotypes account for about 80% to 90% of cervical cancers

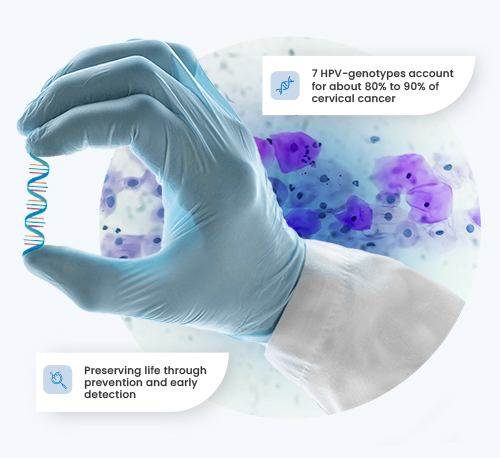
Preserving life through prevention and early detection
Global Diagnostics Labs
A high-complexity CLIA and CAP-certified laboratory
A state-of-the-art diagnostic laboratory that specializes in cutting-edge molecular assays and disease prevention.
PROOFER 7 HPV-mRNA E6 and E7 Biomarker Test
Stratifying the risk
Our confirmatory tests indicate if an active Hr-HPV infection is putting you at a higher risk of developing precancerous cervical lesions.
PROOFER 7 HPV-mRNA E6 and E7 Biomarker Test
Exceptional clinical utility
We have tested over 100,000 samples to prove our test’s ability to help women avoid cervical cancer.

Proofer 7 HPV-mRNA E6 and E7 Biomarker Test
Improved protection against cervical cancer
Human papillomaviruses (HPV) have been identified as the cause of almost all cervical cancers. 14-HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) have been identified as high-risk (Hr-HPV) for cervix cancer, but even among them, risk varies. Two genotypes, HPV 16 and HPV 18, cause about 60-75% of cervical cancer, and HPV 31, 33, 45, 52, and 58 causes about 20%.
Knowledge of the HPV mRNA genotype status dramatically assists in the clinical decision-making of a patient’s risk of having disease now or shortly after, and in choosing the next appropriate management step.
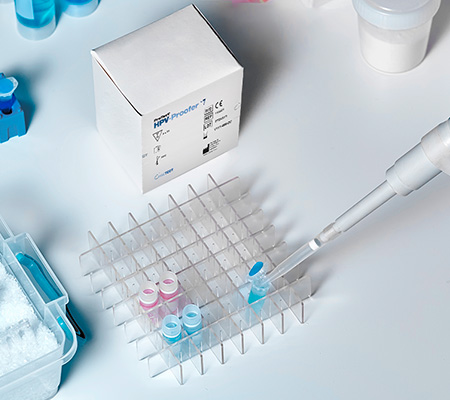
We offer clinical service testing at our CLIA and CAP-certified laboratory
The urgent need to more accurately identify the women who are warranted for colposcopy is highly addressed by clinicians to reduce unnecessary interventions.
OUR PRODUCTS
In vitro molecular reagents
Molecular reagents: Our proprietary RNA-guided molecular genetic technology makes use of multifunctional molecular biomarkers
Sample Preparation solutions: High-performance laboratory sample prep reagents are compatible with our flagship products as well as other brands
IN-VITRO mRNA TECHNOLOGY
WHY HPV E6 AND E7 mRNA IN TRIAGE FOR HPV POSITIVES
01
Higher
specificity
Our studies and literature suggest that HPV E6 and E7 mRNA has higher specificity and positive predictive value as compared with HPV DNA[1].
02
Less referrals and
overtreatment
Proofer 7 HPV-mRNA E6 and E7 technology can serve as a better triage test than HPV DNA to reduce colposcopy referral in both ASC-US and L-SIL. It is also more efficient than cytology for the triage of HPV DNA-positive women[2].
03
Compatible with HPV
self-sampling
In contrast to cervical cytology, triage by molecular biomarkers allows HPV self-sampling. Self-sampling offers excellent potential to increase the participation of hard-to-reach women, thus improving cervical cancer prevention.
TESTIMONIALS
TESTIMONIALS









